
The GO-DACT is an investigator’s initiative trial, in patients with psoriatic arthritis and active dactylitis, which aims to contribute to the clarification of the therapeutic algorithm of dactylitis as a clinical manifestation associated with a worse prognosis.
This is a multicenter, randomized, double-blind, placebo-controlled, phase IIIb trial, which assesses for the first time the comparative efficacy of a TNF inhibitor (golimumab) in combination with MTX versus MTX monotherapy, in improving the dactylitis and enthesitis, in patients with psoriatic arthritis naïve to MTX and biotechnological therapies. The primary endpoint is defined as the variation in the Dactylitis Severity Score (DSS) between the baseline and week 24 of treatment. As secondary endpoints were included the variation in the Leeds Dactilytis Index (LDI) and in the imaging score of dactylitis assessed by magnetic resonance at 24 weeks, as well as the proportion of patients who reached DSS 20, 50 and 70 and LDI 20, 50 and 70 in this timepoint, two innovative indexes developed within the scope of this essay.
Leeds Enthesitis Index, compound indexes and of psoriatic arthritis activity response, as well as indexes related to skin and nail involvement by psoriasis, were additionally evaluated. Reuma.pt worked as a database for this trial, and some of the screens created, such as the DSS screen, later became part of the Reuma.pt clinical practice protocol for patients with psoriatic arthritis.
In the context of this trial, a randomization system was also developed to guarantee equity in the probability of each patient receive the medication allocated to each treatment arm of the trial; a system of therapy management called Reuma.pt-Farmácias, which allows, among other functions, receiving the experimental medication and dispensing it to patients; these tools are available for future use in other clinical trials.
GO-DACT had the collaboration of 11 Rheumatology centers nationwide and reached its primary endpoint demonstrating for the first time that the combination of a TNF inhibitor (golimumab) and methotrexate is superior to methotrexate alone in improving dactylitis active psoriatic disease assessed by DSS and LDI. Consistently, patients treated with this therapeutic combination achieved DSS 50, 70 and LDI 20/50 and 70 response rates significantly higher than patients under MTX monotherapy.
Vieira-Sousa E, Alves P, Rodrigues AM, Teixeira F, Tavares-Costa J, Bernardo A, Pimenta S, Pimentel-Santos FM, Gomes JL, Aguiar R, Pinto P, Videira T, Catita C, Santos H, Borges J, Sequeira G, Ribeiro C, Teixeira L, Ávila-Ribeiro P, Martins FM, Canhão H, McInnes IB, Ribeiro RM, Fonseca JE. GO-DACT: a phase 3b randomised, double-blind, placebo-controlled trial of GOlimumab plus methotrexate (MTX) versus placebo plus MTX in improving DACTylitis in MTX-naive patients with psoriatic arthritis. Ann Rheum Dis. 2020 Apr;79(4):490-498. doi: 10.1136/annrheumdis-2019-216500. PMID: 32193187; PMCID: PMC7147178.
Vieira-Sousa E, Canhão H, Alves P, Rodrigues AM, Teixeira F, Tavares-Costa J, Bernardo A, Pimenta S, Pimentel-Santos F, Gomes JL, Aguiar R, Videira T, Pinto P, Catita C, Santos H, Borges J, Sequeira G, Ribeiro C, Teixeira L, Ávila-Ribeiro P, Martins FM, Ribeiro RM, Fonseca JE. The GO-DACT protocol: a multicentre, randomized, double-blind, parallel-group study to compare the efficacy of golimumab in combination with methotrexate (MTX) versus MTX monotherapy. Acta Reumatol Port. 2018 Apr-Jun;43(2):80-92. English. PMID: 30091952.
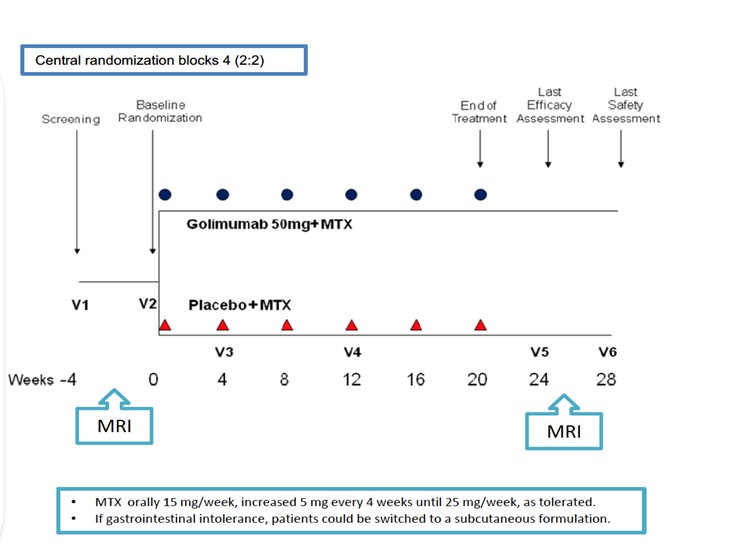
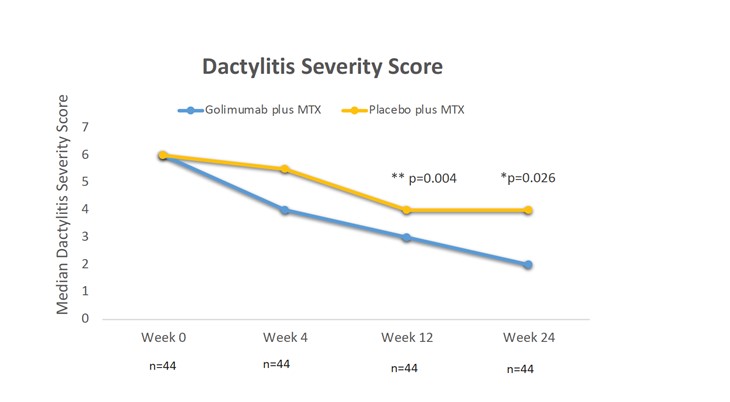
DSS: Dactylitis Severity Score GLM: golimumab; MTX: methotrexate; n: number


